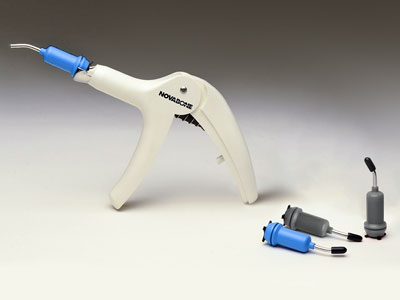
Putty received its FDA approval for dental indications in Feb 2007 & CE approval in November 2007. Putty allows easier manipulation due to its format and eliminates the need for any preparation prior to placement. NB Putty offers clinicians various delivery systems (Shells, Cartridges & Syringes) in various sizes with benefits of consistent, reliable bone regeneration. Built on a Calcium Phosphosilicate (CPS) platform, NB Putty demonstrates superior performance characteristics that are a result of multiple physical & chemical interactions: “Osteostimulation”.
Features
- Unique Dispensing Systems
- Osteostimulation & Osteoconduction
- Cohesion & Graft Retention
- Clot Stabilization
- 100% Synthetic
- Radiodense
- Room Temperature Storage
- 4 Year Shelf-life
Typical uses
- Immediate implant Surgeries
- Extraction Sites
- Sinus Elevations
- Cystic Defects - Apicoectomies
- Cranio-maxillofacial defects (CMF)
- Ridge Augmentations (in combination with morsels)

 Novabone Putty EN
Novabone Putty EN
 Posters and Publications
Posters and Publications